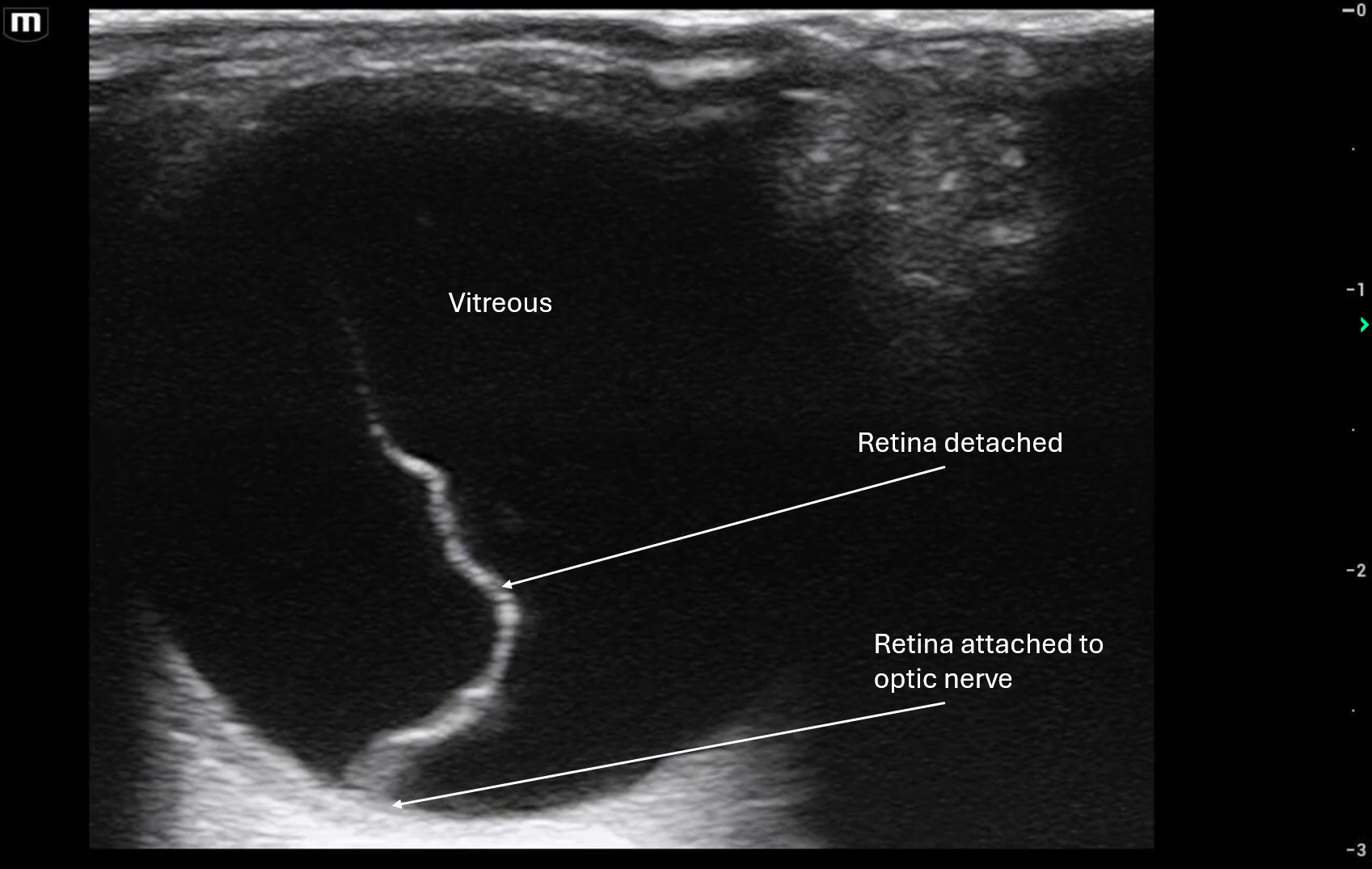
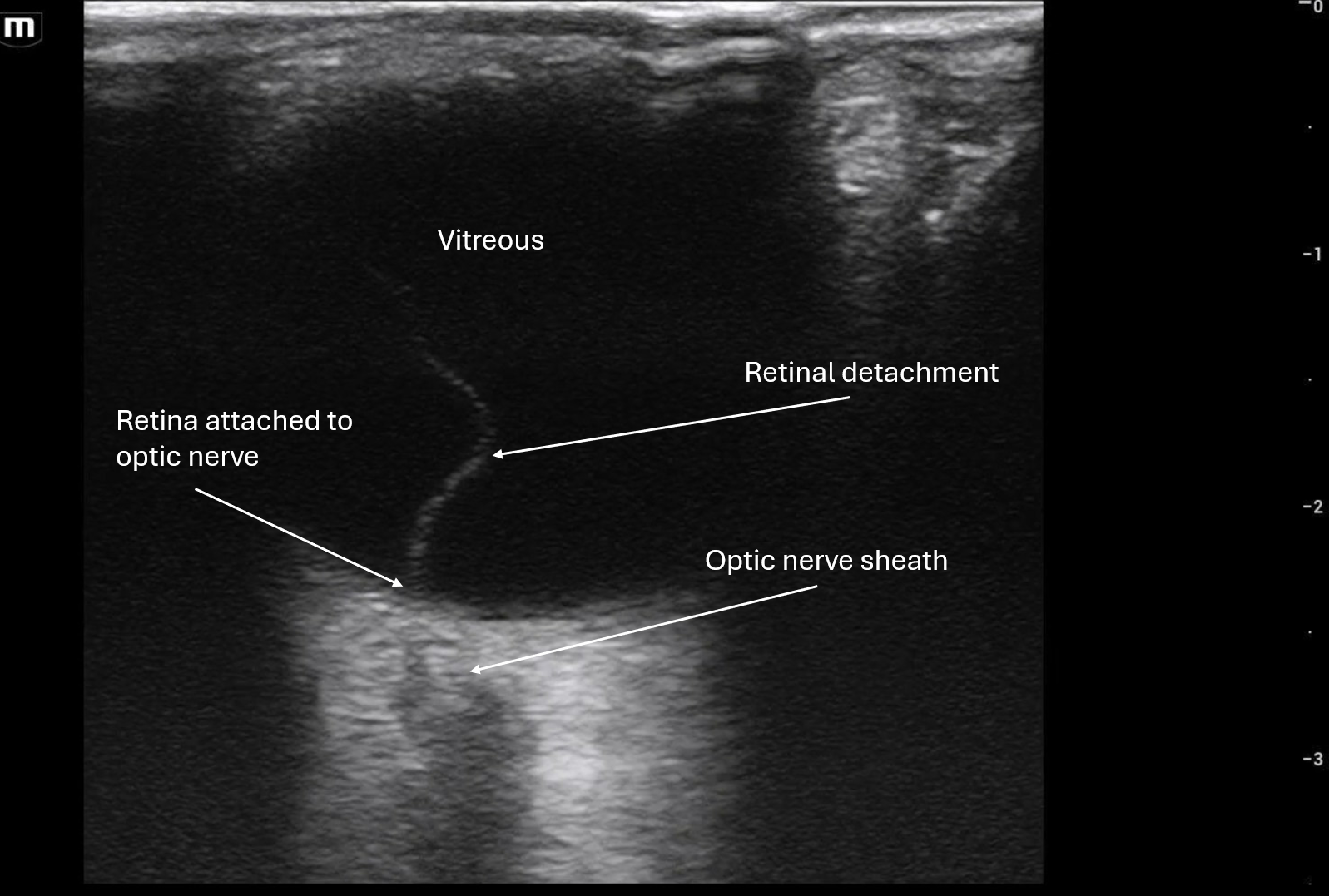
71 y/o male with PMHx of GERD, presents to the ED via EMS found on floor at bottom of stairwell likely from fall with unknown down time. Patient with bilateral racoon eyes, complaining of right arm pain with right elbow deformity, sling in place. Patient is hard of hearing but patient's family at bedside.
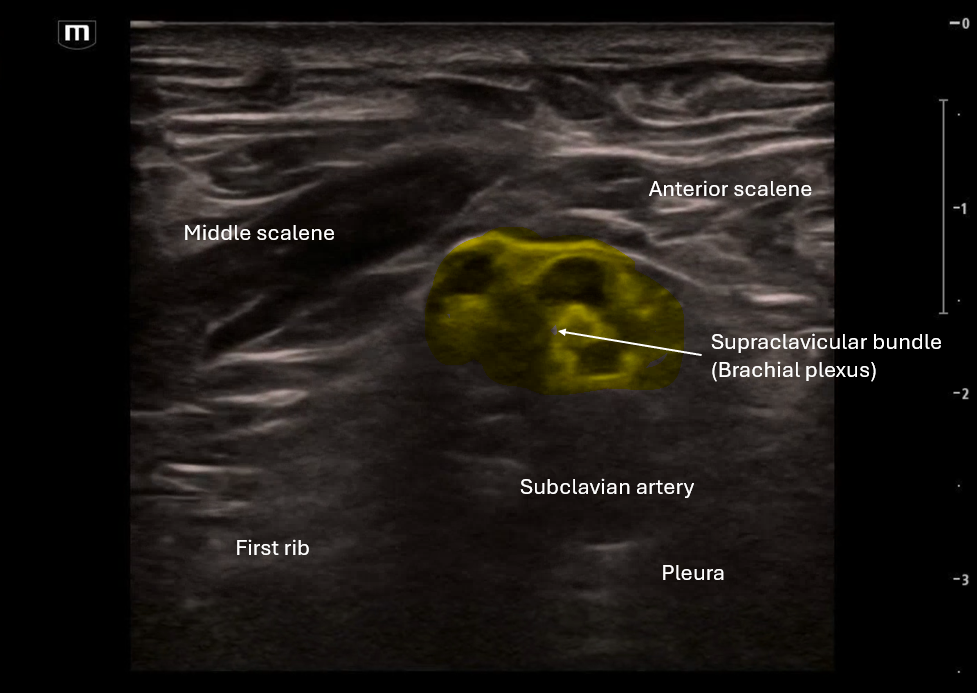
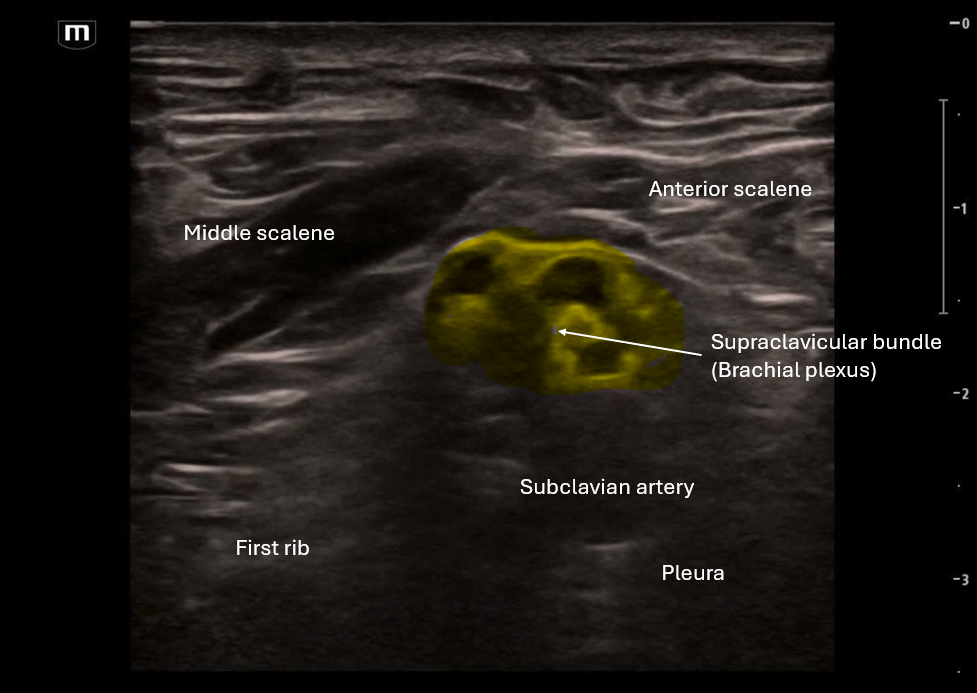
In the first clip we are scanning through to look at the anatomy, this is a sister block to the interscalene and so some of the landmarks are similar with the middle scalene and anterior scalene on either side, but instead of the "stop light" morphology that we see in interscalene block, here we see more of a "bundle of grapes" look. (featuring amazing art from US fellow alumni Dr. Jessie Chen!)
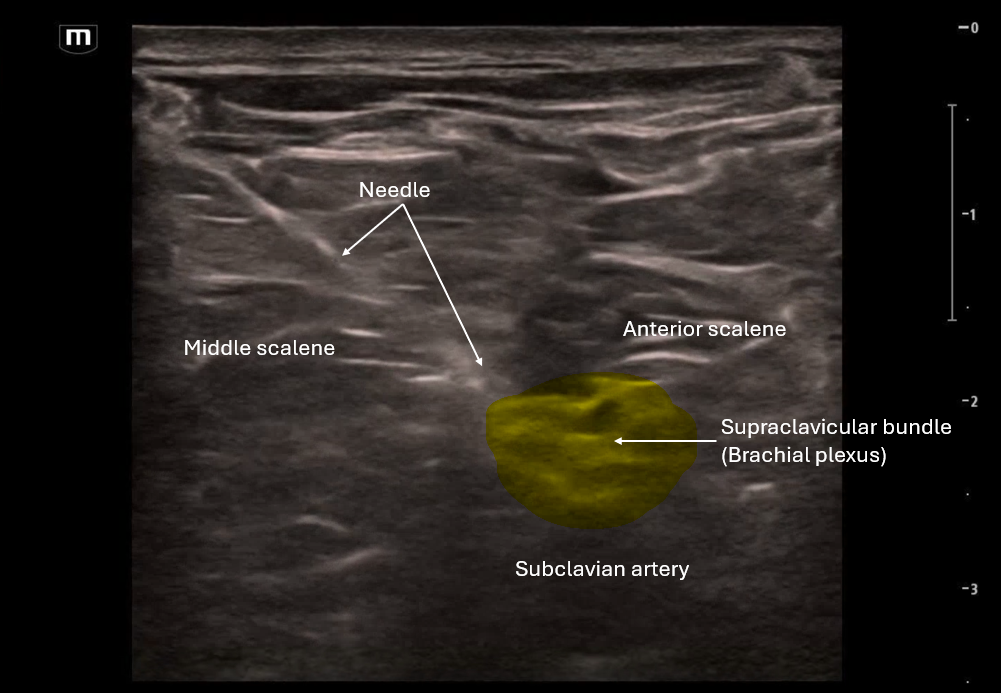
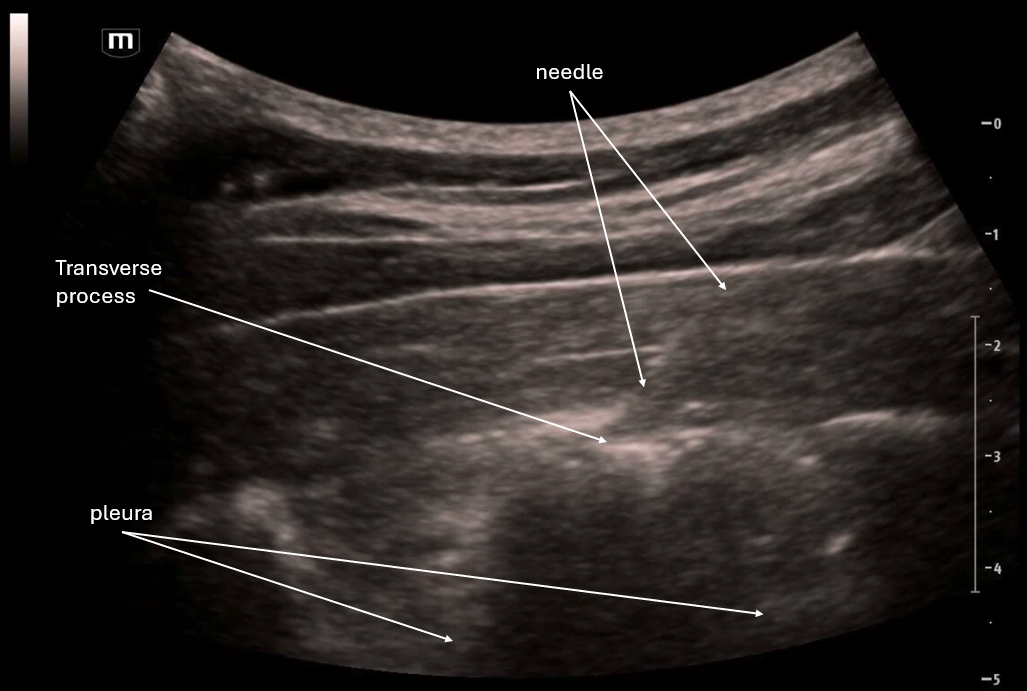
In the 2nd clip we visualize the needle's trajectory as it aims for that supraclavicular bundle.
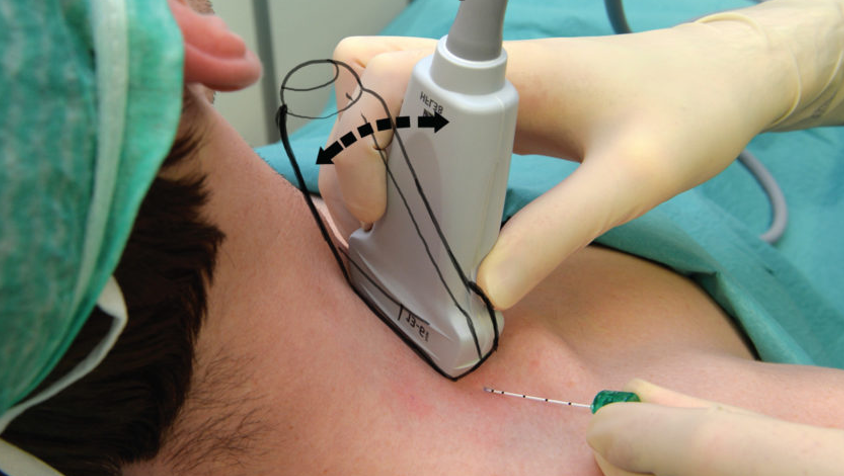
Positioning the patient's head to turn to the left as much as possible is important in order to expose the area around the clavicle.
In the 3rd clip we visualize about 10cc of anesthetic being injected around the bundle. Since this was for a quick procedure we used lidocaine 1%. Always remember to calculate the safe amount of anesthetic for each patient you do a nerve block for!
The team was able to reduce the right shoulder successfully!
POCUS Pearls for Supraclavicular Nerve Blocks:
Indications include upper extremity fractures (distal to mid-humerus), elbow dislocations, forearm lacerations
Use linear probe positioned transverse just above clavicle; head turned away
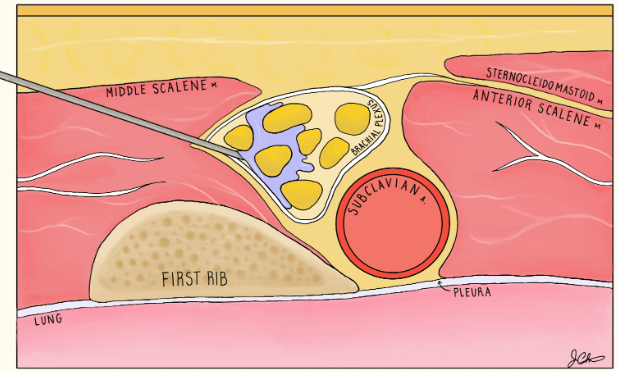
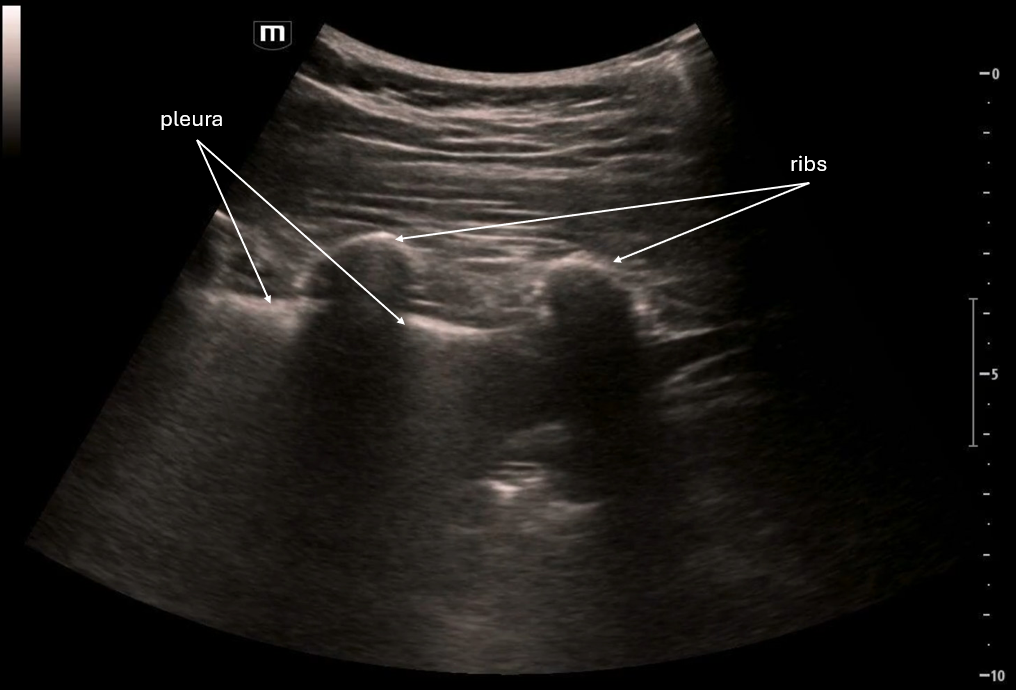
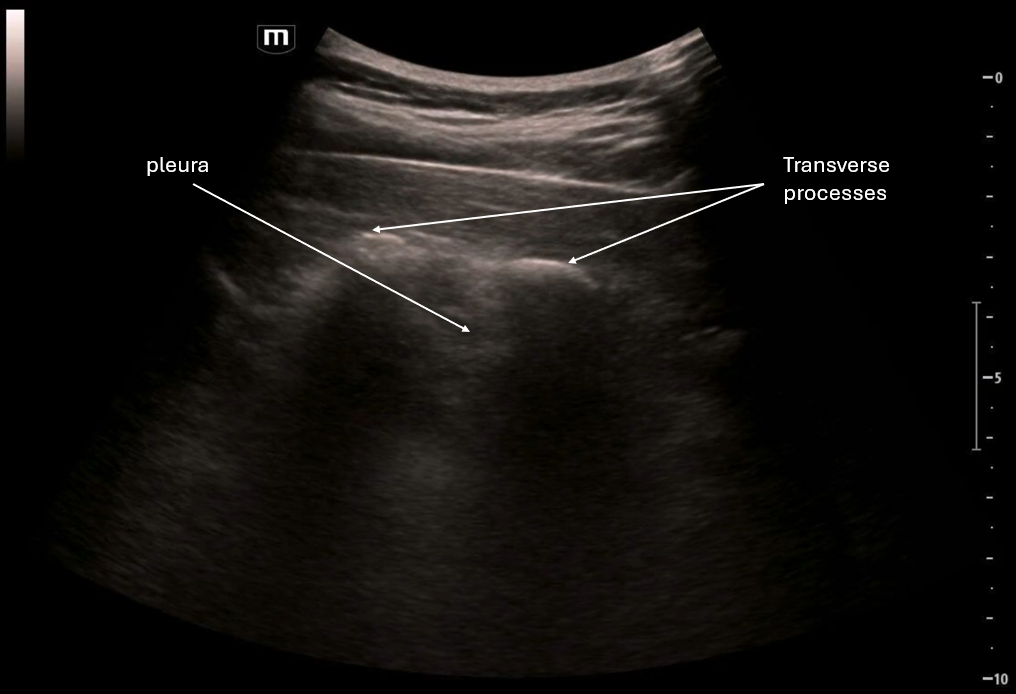
Find artery medial with “bundle of grapes” lateral and visualize the rib deep
Rib is the safety backstop and visualize the pleura deep and medial
In-plane approach with needle entering lateral towards medial
Visualize tip at all times; hydrodissect 1–2 mL before injecting anesthetic
Inject 10-15 mL for circumferential spread around the bundle